DURING the summer of 2021, the UK Medical Freedom Alliance (UKMFA) became concerned about an unseasonal and unprecedented rise in excess mortality in Scotland, seen in the official ONS data. The onset of this rise varied across different age cohorts, coinciding with a timepoint of around 12 weeks following the Covid vaccine rollout to each cohort. It was, notably, unrelated to deaths from COVID-19.
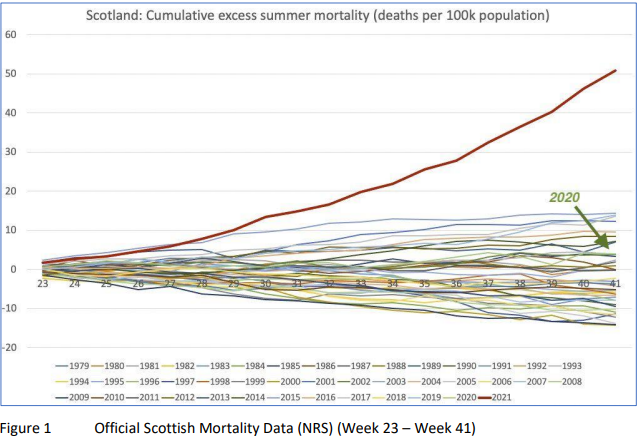
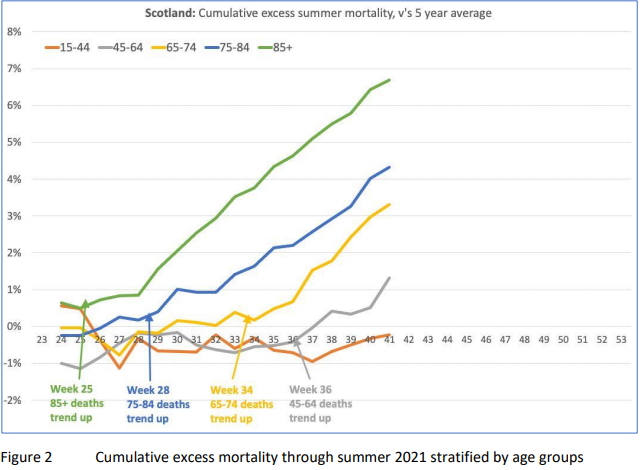
At the end of October 2021, we wrote an urgent open letter to Public Health Scotland, presenting the data and asking them to investigate the possible link with the Covid vaccines and take appropriate action. After several months, and a chain of frustrating correspondence between UKMFA and statisticians at the Scottish Government Health and Social Care Analysis, the recommendation was to put our concerns to the Medicines and Healthcare Regulatory Agency (MHRA). In July 2022, we sent a Freedom of Information (FOI) request to the MHRA, asking whether they had investigated this trend. We received an unsatisfactory reply in September 2022, in which we were told the MHRA were proactively and closely monitoring Covid vaccine safety but did not hold the data requested.
As part of their Covid vaccine safety surveillance strategy, the MHRA had pledged to carry out epidemiological studies when indicated. We therefore sent a further FOI request in October 2022, asking them what criteria they had set to trigger an epidemiological study. We argued that an increase in excess deaths should be a strong indicator for such studies. We also requested documents and discussions relied upon to decide how many fatal outcomes reported through the Yellow Card system were caused by a Covid vaccine and any documents or discussions referring to rapid cycle analysis or targeted active monitoring, in the context of safety surveillance of these products.
Despite our repeatedly chasing up the MHRA, they failed to respond to this second FOI, and in April 2023 we lodged a formal complaint with the Information Commissioner’s Office (ICO). The ICO upheld our complaint, ruling that the MHRA had breached section 10(1) of the FOIA by failing to provide a valid response to the request within the statutory time frame of 20 working days. The Commissioner instructed the MHRA to provide a substantive response to our FOI request within 35 calendar days, warning that failure to comply may be dealt with as a contempt of court.
The MHRA finally responded in June 2023 but, disappointingly, their response was grossly inadequate with no acknowledgement of the gravity of our concern and no supporting documents provided. They admitted to holding some of the information requested but claimed an exemption due to the cost involved to release it being over £600. However, they did admit that they had NO set criteria to trigger an epidemiological study regarding a safety concern. They also disclosed that they do not ascribe causality to individual reported deaths and do not make decisions on vaccination policy.
UKMFA responded by email, requesting an internal review on the grounds that:
‘We have received no documentation relating to any of our questions – emails, minutes of meetings etc – that we requested. We also wish to challenge your use of the Section 12 FOIA exemption. We take no comfort in your glib, but entirely unsubstantiated claim that ‘It is important to note that most people receive vaccinations without having any serious side effects’, especially given your previous admission that ‘we do not assign causality (i.e. whether the patient’s death was caused by the vaccine) at the level of individual reports’ . . . [so] the MHRA appears to have no system of active safety monitoring in place that would even identify a safety signal, let alone to then act on it to protect the public.’
The MHRA Internal review, received by UKMFA in August 2023, made it apparent that the agency has no interest pursuing an active safety surveillance or investigating potential safety signals arising from the rollout of Covid vaccines.
Two quotes taken from the MHRA responses are particularly alarming, clearly illustrating the contempt in which the regulatory agency in the UK holds those who have suffered as a result of taking this novel, gene-based product:
1. ‘Adverse events, including events that are reported in temporal association with vaccines, occur naturally in the population.’
This is an extraordinary statement implying that any deaths temporally associated with Covid vaccination may be explained away as coincidental – especially considering that ‘naturally occurring’ coincidental adverse events were never considered when counting deaths from COVID-19. In addition. the term ‘adverse event’ is not usually applied to illnesses that arise naturally, only to the consequences of an intervention. This statement brings into question the ability of this agency to fulfil their remit to protect public health by monitoring for and acting on safety signals.
2. ‘It is important to note that most people receive vaccinations without having any serious side effects.’
This chilling statement reveals that the MHRA do not see their responsibility to ensure safety of all people, but only ‘most people’. The MHRA must urgently clarify what they mean by ‘most people’ and what they see as their responsibility (if any) to those who are not in this category. It is unethical to justify the failure to take regulatory action, following the reported deaths of over 2,500 people, with the assertion that most people did not die.
Dissatisfied with the MHRA internal review, we appealed to the ICO to investigate our concerns that the MHRA had failed to respond to our FOI request in any meaningful way and also failed to reassure that they carry out their assigned task of Covid vaccine safety monitoring in a reliable and responsible manner. Our complaint was sent on November 3, 2023, but astonishingly the ICO informed us that due to ‘undue delay’ of more than six weeks in submitting our complaint (following the MHRA internal review notification), they were closing the case and we would need to submit a new FOI to the MHRA. This we have done. In light of the MHRA continuing recommendation of Covid vaccines and boosters, including to pregnant women, we submitted a new FOI on November 20, this time requesting disclosure of documents that support their assurance of safety of the Covid vaccines, specifically relating to carcinogenicity, autoimmunity and genotoxicity.
After a long and tortuous process spanning nearly 18 months, as it stands there will be no further investigation of the MHRA’s failure to conduct the rigorous safety surveillance required to protect the public from potential harm. The casual and callous attitude of our regulatory agency towards each person who has died or come to serious harm, as a result of a product approved and promoted by themselves, must be brought to public attention.
It is time for the public to demand that the MHRA fulfil their assigned responsibility proactively to monitor safety and take a precautionary approach by investigating any possible signal of harm. Their duty is to protect public health, not to enable Big Pharma.